KKDIK Update: Procedures and Principles Have Been Determined!
The "Procedures and Principles on the Implementation of the Regulation on Registration, Evaluation, Authorization, and Restriction of Chemicals," which clarifies the registration processes of the KKDIK (Turkey REACH) Regulation and provides a roadmap for all stakeholders, has been officially published. Critical deadlines and important obligations have now been finalized.
As Chemvisor, your dedicated consultant closely monitoring the process, we have compiled the most significant highlights for you:
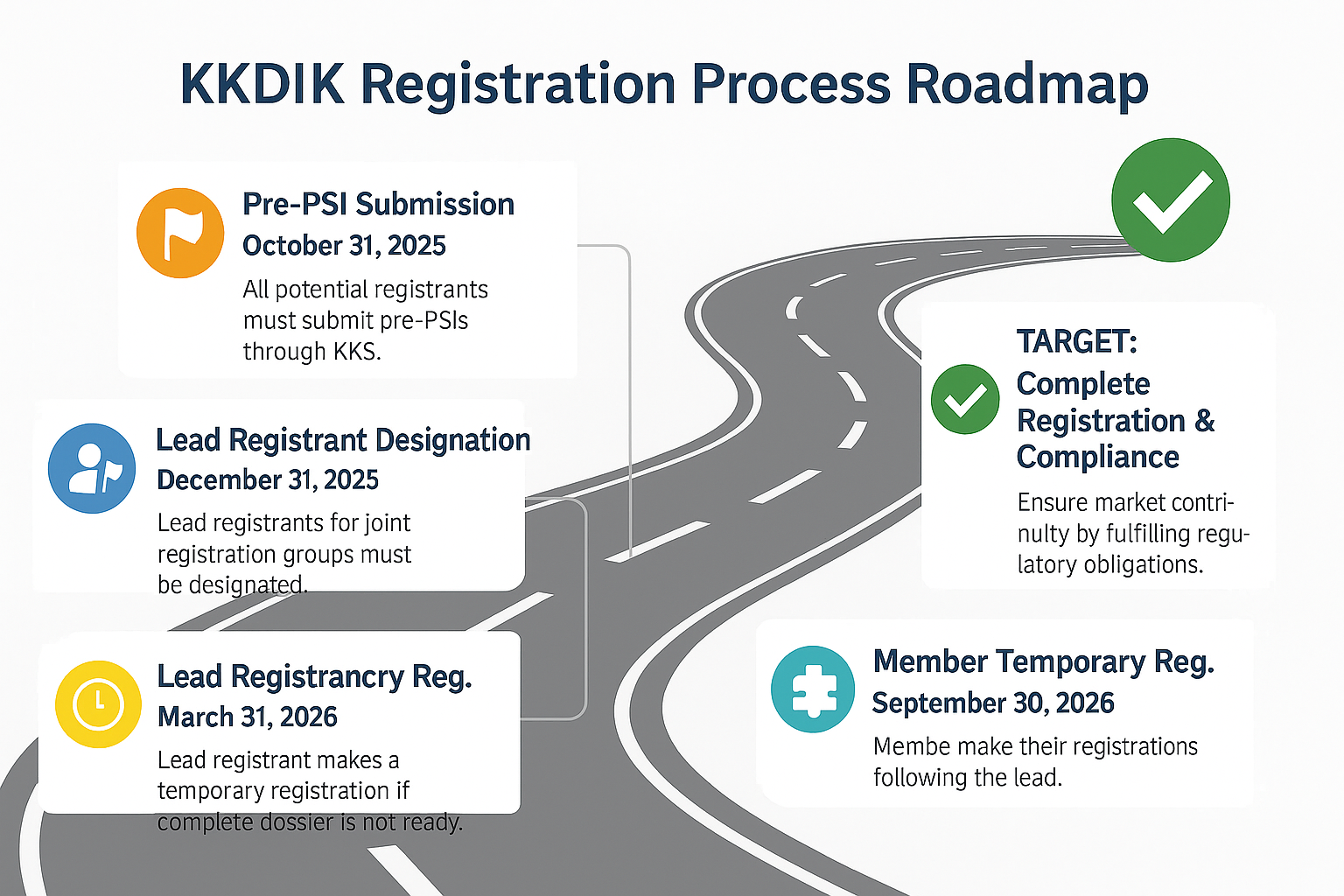
Be Sure to Mark These Critical Dates on Your Calendar!
- October 31, 2025: Deadline for Pre-SIEF Notification! All potential registrants who manufacture or import substances in quantities of 1 ton or more per year are required to submit their pre-SIEFs through the Chemical Registration System (KKS) by this date to participate in the Substance Information Exchange Forum (SIEF).
- December 31, 2025: Deadline for Appointing Lead Registrants! This has been set as the final date for the selection of lead registrants, a key step in the joint registration process. While the process is primarily based on voluntary participation, the Ministry may intervene if a lead registrant is not appointed by the specified deadline.
- March 31, 2026 & September 30, 2026: "Temporary Registration" Mechanism and Dates A "Temporary Registration" option has been introduced for companies facing difficulties in preparing the full registration dossier. Lead registrants can complete their temporary registrations by March 31, 2026, and member registrants can do so by September 30, 2026, following the lead's submission.
Other Important Developments:
- One Substance, One Registration Principle: It has been re-emphasized.
- CAS (KDU) Responsibility: It is now mandatory for temporary registration data to be entered into the system by a certified Chemical Assessment Specialist (CAS / KDU).
- SDS Obligations: Safety Data Sheets (SDS), prepared in accordance with KKDIK Annex-II, must be uploaded to the Ministry's dedicated system. The SDS must also include the certificate number and contact details of the CAS who prepared it.
While these Procedures and Principles accelerate the KKDIK compliance process, they also place significant responsibilities on industry. The very near deadlines make it critical to plan the necessary steps without delay.
How We Can Support You as Chemvisor
As Chemvisor, with our deep experience gained since the early days of the EU REACH Regulation and our expert team, we are by your side in this challenging process. We offer you tailored solutions at every stage of the KKDIK compliance process:
- Comprehensive Portfolio Analysis and Determination of Your Compliance Strategy
- Only Representative Service for Non-Turkish Entities
- Submission and Management of Your Pre-SIEF and C&L Notifications
- SIEF Management, Consortium Communication, and Leadership Support
- Technical and Administrative Preparation of Lead and Member Registration Dossiers
- Preparation and Updating of KDU-Approved Safety Data Sheets (SDS) Compliant with KKDIK Annex-II
For detailed information and your compliance, please contact us.
info@chemvisor.com.tr ? www.chemvisor.com.tr
Official Announcement: https://lnkd.in/dgHrbgYM